Authors: Marta Sevilla (INCAR-CSIC), Noel Díez (INCAR-CSIC) and Antonio B. Fuertes (INCAR-CSIC)
Article published on: ChemSusChem
ChemSusChem 2021, 14, 94 – 117
Abstract
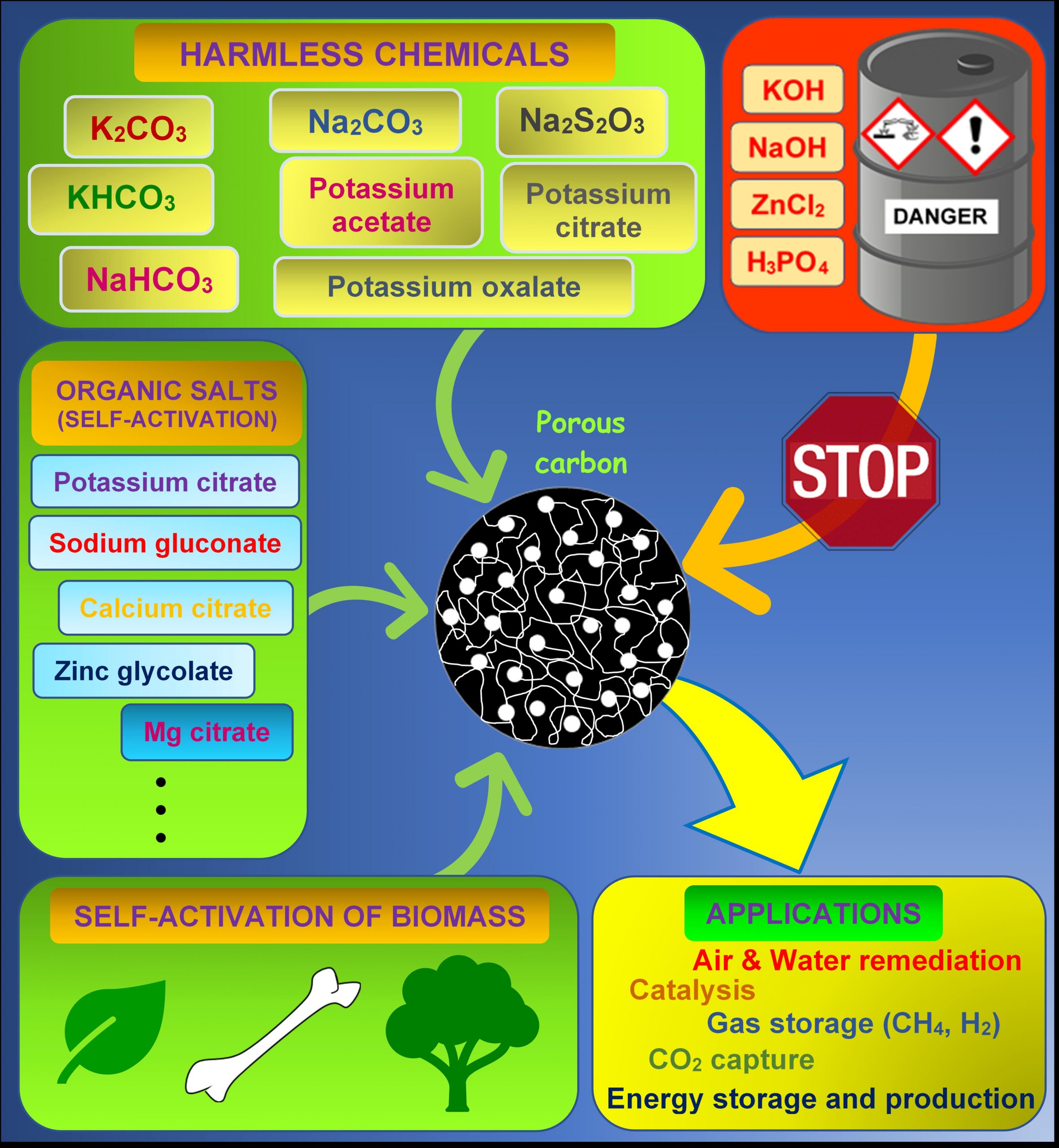
The preparation of porous carbons attracts a great deal of attention given the importance of these materials in many emerging applications, such as hydrogen storage, CO2 capture, and energy storage in supercapacitors and batteries. In particular, porous carbons produced by applying chemical activation methods are preferred because of the high pore development achieved. However, given the environmental risks associated with conventional activating agents such as KOH, the development of greener chemical activation methodologies is an important objective. This Review summarizes recent progress in the production of porous carbons by using more sustainable strategies based on chemical activation. The use of less-corrosive chemical agents as an alternative to KOH is thoroughly reviewed. In addition, progress achieved to date by using emerging self-activation methodologies applied to organic salts and biomass products is also discussed.
Many works gathered in this review correspond to the “Grupo de Materiales Porosos Funcionales” at INCAR, which has been involved in the last years in the development of more environmentally sound approaches for the production of porous carbon materials with advanced properties for their use in electrochemical energy storage applications.
Summary and outlook
There is no doubt that porous carbons play a key role in many emergent applications, including electrochemical energy storage/production, carbon capture and gas storage (e.g., H2 or CH4), and still play a leading role in traditional applications such as catalysis and water/gas purification. Traditional activation processes, especially chemical activation procedures, are based on the use of (highly) corrosive, toxic substances. This not only poses significant restrictions on equipment and safety protocols, but also represents a serious environmental threat. This has spurred the search for greener chemical activating agents, as well as the development of novel activation procedures. However, it should be noted that potassium hydroxide is still massively used at the laboratory scale, as it allows the synthesis of high-performing activated carbons with advanced properties. It is the authors’ opinion that future efforts are mandatory from the scientific community to replace potassium hydroxide, as its use is quite unrealistic from the industrial point of view.